Ask for a reprint
email :
* Give your email
2011
ACL
|
G.L.Athens, D.Kim, J.D.Epping, S.Cadars, Y.Ein-Eli, B.F.Chmelka, 'Molecular Optimization of Multiply-Functionalized Mesoporous Films with Ion Conduction Properties', J. Am. Chem. Soc. 133 16023–16036 (2011) doi:10.1021/ja2038529
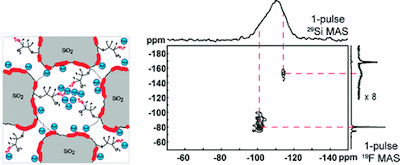
Sequential processing of multiply-functionalized mesoporous films is shown to yield materials that are compositionally and structurally heterogeneous on mesoscopic and molecular length scales, both of which must be controlled to optimize macroscopic ion-conduction properties. Cubic mesoporous silica films prepared from strongly acidic solutions were subsequently functionalized under highly alkaline conditions to incorporate hydrophilic aluminosilica surface moieties, followed by non-aqueous conditions to introduce perfluoro-sulfonic-acid surface groups. Such sequential combination of individually incompatible steps yielded stable mesoporous films with high surface hydrophilicities and strong acid functionalities that exhibited high proton conductivities (ca. 910-2 S/cm) at elevated temperatures (120 oC). Molecular, mesoscopic, and macroscopic properties of the multiply-functionalized films were monitored and correlated at each stage of the syntheses by nuclear magnetic resonance (NMR) spectroscopy, small-angle X-ray scattering (SAXS), transmission electron microscopy (TEM), elemental analysis, adsorption, and ion conductivity measurements. In particular, variable-temperature solid-state two-dimensional (2D) 27Al{1H}, 29Si{1H}, 27Al{19F}, and 29Si{19F} HETeronuclear chemical-shift CORrelation (HETCOR) NMR spectra reveal separate surface adsorption and grafting sites for the different functional surface species within the mesopore channels. The hydrophilic aluminosilica and acidic fluoro-group loadings and interaction sites are demonstrated to be strongly affected by the different synthesis and functionalization treatments, which must be separately and collectively optimized to maximize the proton conductivities.
|
|