Ask for a reprint
email :
* Give your email
2020
ACL
|
N.Dubouis, A.Serva, R.Berthin, G.Jeanmairet, B.Porcheron, E.Salager, M.Salanne, A.Grimaud, 'Tuning water reduction through controlled nanoconfinement within an organic liquid matrix', Nature Catalysis 3 656–663 (2020) doi:10.1038/s41929-020-0482-5
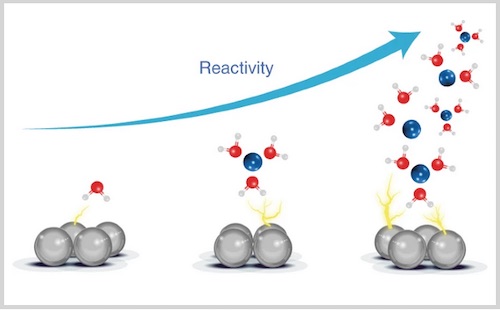
The growing hydrogen economy requires accelerating the hydrogen evolution reaction. The water dissociation step (Volmer step) has been proposed as a main kinetic limitation, but the mechanisms at play in the electrochemical double-layer are poorly understood. This is due to the dual role of water: it acts both as a reactant and as a solvent. Here we propose to confine water inside an organic liquid matrix in order to isolate the sole role of water as a reactant. We observed the formation of aqueous-rich nanodomains, whose size can be tuned by changing the supporting electrolyte and found that the reactivity of the system varies significantly with its nanostructure. Depending on the conditions, the reactivity is dominated by either the strength of short-range cation–water interactions or the formation of long chains of water molecules. Understanding this paves the way towards the development of more efficient and selective electrocatalysts for water, CO2, O2 or N2 reduction.
|
|