Ask for a reprint
email :
2020
ACL
|
J.Fan, V.Sarou-Kanian, X.Yang, M.Diaz-Lopez, F.Fayon, X.Kuang, M.J.Pitcher*, M.Allix*, 'La2Ga3O7.5: a metastable ternary melilite with a super-excess of interstitial oxide ions synthesized by direct crystallization of the melt', Chem. Mat. 32 9016-9025 (2020) doi:10.1021/acs.chemmater.0c03441
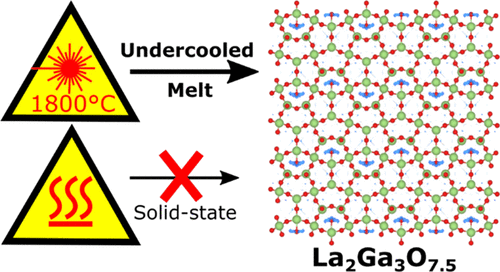
The La1+xAE1-xGa3O7+x/2 melilite family (AE = Ca, Sr, Ba, and 0 x 0.64) demonstrates remarkable oxide ion
conductivity due to the ability of its layered tetrahedral [Ga3O7+x/2] network to accommodate and transport interstitial oxide ions (Oint).
Compositions x > 0.65 with very high Oint concentrations (referred to here as “super-excess” compositions) have the potential to
support correspondingly high ionic conductivities, but have never before been accessed due to the limitations of conventional solidstate
ceramic synthesis. Here we report that fully-substituted La2Ga3O7.5 (x = 1) melilite ceramics can be synthesized by direct
crystallization of an under-cooled melt, demonstrating that super-excess compositions are accessible under suitable nonequilibrium
reaction conditions. La2Ga3O7.5 is stable up to 830 °C and exhibits an ionic conductivity of 0.01 S.cm-1 at 800 °C, three orders of
magnitude higher than the corresponding x = 0 end-member LaSrGa3O7, and close to the range exhibited by the current best-in-class
La1.54Sr0.46Ga3O7.23 (0.1 S.cm-1). It crystallizes in an orthorhombic √2a x √2a x 2c expansion of the parent melilite cell, in space group
Ima2, with full long-range ordering of Oint into chains within the [Ga3O7.5] layers. The emergence of this chain-like (1D) ordering
within the 2D melilite framework, which appears to be an incipient feature of previously reported partially-ordered melilites, is
explained in terms of the underlying hexagonal topology of the structure. These results will enable the exploration of extended
compositional ranges for the development of new solid oxide ion electrolytes with high concentrations of interstitial oxide charge
carriers.
|
|