Ask for a reprint
email :
* Give your email
2020
ACL
|
A.Luna-Triguero, A.Sławek, R.Sánchez-de-Armas, J.J.Gutiérrez-Sevillano, C.O.Ania, J.B.Parra, J.M.Vicent-Luna, S.Calero, 'π-Complexation for olefin/paraffin separation using aluminosilicates', Chem. Eng. J. 380 122482 (2020) doi:10.1016/j.cej.2019.122482
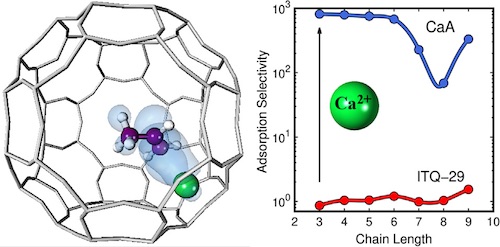
The purification of the α-olefins though challenging, is mandatory step for their use in the chemical industry. Since adsorptive separation using zeolites is one of the most promising alternatives for olefin/paraffin separation in terms of energy efficiency, we use a combination of experiments and molecular simulations to study the effect that the topology and chemical composition of the zeolite exert on the purification of olefins. To this aim we developed an effective potential for the cations with the double bond of the olefins. The potential parameters were validated with our experimental adsorption isotherms and isobars of propylene and 1-hexene. We performed an extensive study of propane/propylene separation in more than 200 all silica zeolites and several aluminosilicates. We also performed DFT and classical optimization of the structures, obtaining the minimum energy of a given chemical composition and topology, which is key factor for the adsorption mechanisms. DFT calculations also allowed the analysis of binding energies and binding geometries of propane and propylene in NaY and LTA5A. We discussed the effect exerted by the cations on the separation performance of the zeolites. Our study shows that aluminosilicates with calcium cations are the best candidates to separate olefins from paraffins, due to the stronger interaction of the double bond of olefins with these divalent cations.
|
|