Ask for a reprint
email :
* Give your email
2014
ACL
|
L.Lander, M.Reynaud, G.Rousse, M.T.Sougrati, C.Laberty-Robert, R.Messinger, M.Deschamps, J.M.Tarascon, 'Synthesis and electrochemical performance of the orthorhombic Li2Fe(SO4)2 polymorph for Li-ion batteries', Chem. Mat. 26 4178–4189 (2014) doi:10.1021/cm5012845
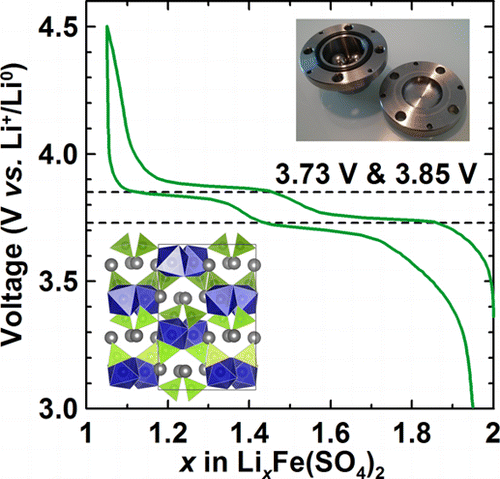
To enhance the safety, cost, and energy density of Li-ion batteries, significant research efforts have been devoted to the search for new positive electrode materials that exhibit high redox potentials and are composed of low-cost, earth-abundant elements. Sulfate chemistry has yielded promising results for iron-based polyanionic electrode materials using the FeIII+/FeII+ redox couple, including the recent discovery of a monoclinic marinite Li2Fe(SO4)2 phase (3.83 V vs Li+/Li0). Here, we report the ball-milling synthesis and electrochemical properties of a new orthorhombic polymorph of Li2Fe(SO4)2, which reversibly reacts with lithium through a two-step redox process (3.73 and 3.85 V vs Li+/Li0) with an overall sustained capacity of about 90 mAh/g. Using similar synthesis conditions, the cobalt-, zinc-, magnesium-, and nickel-based orthorhombic analogues were also obtained, though no electrochemical activity was observed for these phases. Overall, our results demonstrate that polymorphism can play a crucial role in the search for new battery electrode materials and emphasize the need to understand and master synthetic control.
|
|