Ask for a reprint
email :
* Give your email
2018
ACL
|
A.A.Kulbakov, M.Allix, A.Rakhmatullin, A.S.Mikheykin, Yu.Popov, N.V.Smirnova, O.A.Maslova, I.N.Leontyev, 'In-situ investigation of non-isothermal decomposition of Pt acetylacetonate as One-Step size-controlled synthesis of Pt Nanoparticles', Phys. Stat. Solidi A 215 1800488 (2018) doi:10.1002/pssa.201800488
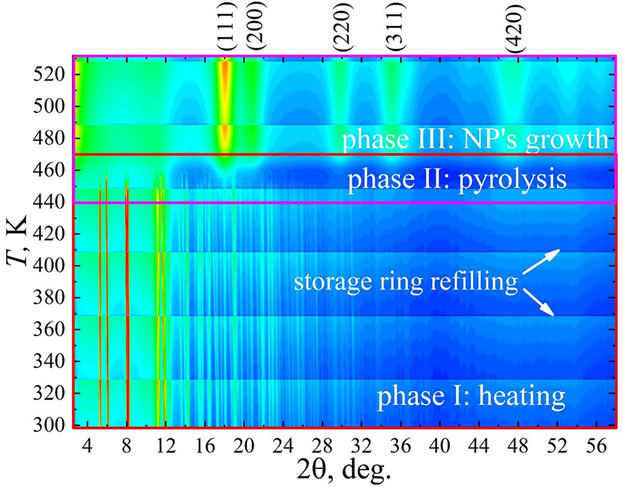
Interest in the development of state‐of‐the‐art methods for synthesis of Pt nanoparticles is dictated by both unique properties of platinum itself and the wide range of platinum nanoparticles applications. Platinum acetylacetonate and platinum nanoparticles produced by the thermal decomposition of Pt(acac)2 are studied via in situ X‐ray diffraction and Raman spectroscopy, thermal gravimetry, scanning (SEM), and transmission electronic microscopy (TEM). The experiments are made at heating rates of 1 and 5 K min−1. A comparative analysis of SEM, TEM, and XRD data highlights the formation of coarse Pt particles with sizes of ≈60–160 nm, which are composed of nanoparticles with dimensions of 1.9 and 4.1 nm for 1 K min−1‐ and 5 K min−1‐heating rates, respectively. The desirable average size of nanoparticles upon their synthesis can thus be achieved by a simple tuning of the heating rate of acetylacetate thermal decomposition. The present results open up the possibility to use a trivial non‐isothermal thermal decomposition method in the synthesis of both supported and unsupported nanoparticles of predetermined size for other chemical elements such as Pd, Ni, Co, etc. from their acetylacetonates.
|
|