Ask for a reprint
email :
* Give your email
2022
ACL
|
Nadia B.Haro-Mares, Juan C.Meza-Contreras, Fernando A.López-Dellamary, Arlette Richaud, Francisco Méndez, Brenda G.Curiel-Olague, Gerd Buntkowsky, Ricardo Manríquez-González, 'Lysine functionalized cellulose for a zwitterion-based immobilization of laccase enzyme and removal of commercial dyes from aqueous media', Surfaces and Interfaces 35 102412 (2022) doi:10.1016/j.surfin.2022.102412
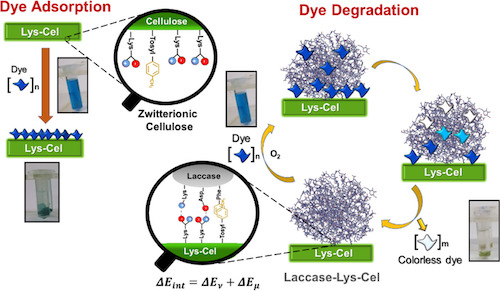
The zwitterion functionalization of cellulose with lysine was achieved in a two-step synthesis. The cellulose modification in each step of the synthesis was corroborated by FTIR and solid state 13C NMR spectroscopy. The zwitterionic character in the modified cellulose was determined by its isoelectric point using a titration curve vs. pH. The dye adsorption performance of the modified cellulose was evaluated with malachite green and indigo carmine solutions. Cellulose modification increases laccase immobilization almost 10-fold compared to unmodified cellulose. Ionic and hydrophobic interactions are involved in immobilization, stabilizing laccase and the zwitterionic cellulose system. A novel theoretical approach of this immobilization is also proposed involving the interaction energies of the specific ionic moieties and supported by hydrophobic interactions shared between the enzyme and the zwitterionic cellulose interface. Moreover, immobilization did not affect the bioactivity of the enzyme, but on the contrary increased it by 29%. The laccase and zwitterionic cellulose system (Laccase-Lys-Cel) was tested against malachite green and indigo carmine in a 96-h experiment. The best removal results of about 93% from aqueous solution were observed for indigo carmine after 1 h and 99% after 72 h. Finally, Laccase-Lys-Cel combines two mechanisms: (1) dye adsorption in the early stages and (2) final dye degradation by the enzyme.
|
|