Ask for a reprint
email :
* Give your email
2020
ACL
|
C.Yang, G.Rousse, K.Svane, P.Pearce, A.Abakumov, M.Deschamps, G.Cibin, A.Chadwick, D.Alves Dalla Corte, H.Hansen, T.Vegge, J.M.Tarascon, A.Grimaud, 'Cation Insertion to Break the Activity/Stability Relationship for Highly Active Oxygen Evolution Reaction Catalyst', Nat. Commun. 11 1378 (2020) doi:10.1038/s41467-020-15231-x
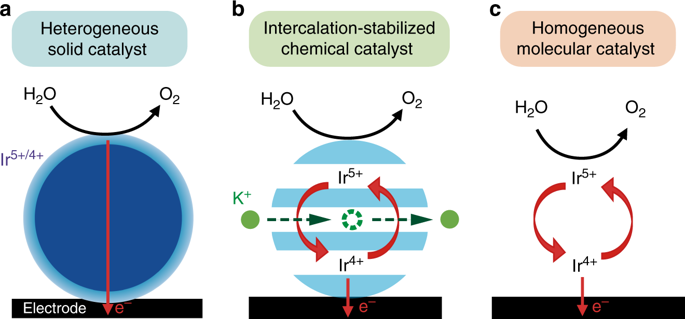
The production of hydrogen at a large scale by the environmentally-friendly electrolysis process is currently hampered by the slow kinetics of the oxygen evolution reaction (OER). We report a solid electrocatalyst α-Li2IrO3 which upon oxidation/delithiation chemically reacts with water to form a hydrated birnessite phase, the OER activity of which is five times greater than its non-reacted counterpart. This reaction enlists a bulk redox process during which hydrated potassium ions from the alkaline electrolyte are inserted into the structure while water is oxidized and oxygen evolved. This singular charge balance process for which the electrocatalyst is solid but the reaction is homogeneous in nature allows stabilizing the surface of the catalyst while ensuring stable OER performances, thus breaking the activity/ stability tradeoff normally encountered for OER catalysts.
|
|