Ask for a reprint
email :
* Give your email
2007
ACL
|
P.Azaïs, L.Duclaux, P.Florian, D.Massiot, M.A.Lillo-Rodenas, A.Linares-Solano, J.P.Peres, C.Jehoulet, F.Béguin, 'Causes of supercapacitors ageing in organic electrolyte', J. Power Sources 171 1046-1053 (2007) doi:10.1016/j.jpowsour.2007.07.001
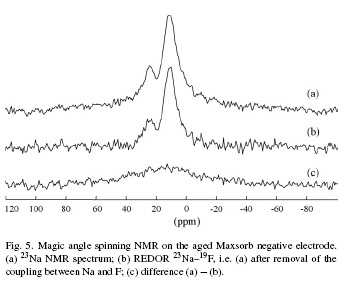
Abstract In order to understand what causes supercapacitors ageing in an organic electrolyte (tetraethylammonium tetrafluoroborate – Et4NBF4 – 1 mol L−1 in acetonitrile), the activated carbon electrodes were characterized before and after prolonged floating (4000–7000 h) at an imposed voltage of 2.5 V. After ageing, the positive and negative electrodes were extensively washed with pure acetonitrile in neutral atmosphere to eliminate the physisorbed species. Then, the carbon materials were dried and transferred without any contact with air to be studied by XPS, 19F NMR, 11B NMR and 23Na NMR. Decomposition products have been found in the electrodes after ageing. The amount of products depends on the kind of activated carbon and electrode polarity, which suggests redox reactions of the electrolyte with the active surface functionality. Nitrogen adsorption measurements at 77 K on the used electrodes showed a decrease of accessible porosity, due to trapping of the decomposition products in the pores. Hence, the evolution of the supercapacitor performance with time of operation, i.e. the capacity decrease and the resistance increase, are due to the decomposition of the organic electrolyte on the active surface of the carbon substrate, forming products which block a part of porosity. The concentration of surface groups and their nature were found to have an important influence on the performance fading of supercapacitors.
|
|