Ask for a reprint
email :
* Give your email
2022
ACL
|
Simon Blotevogel, Mathilde Poirier, Delphine Vantelon, Erwan Chesneau, Charles-E Dutoit, Valérie Montouillout, Franck Fayon, Judit Kaknics, Gautier Landrot, Giuseppe D.Saldi, Jacques Schott, Hervé Vezin, Cedric Patapy, Martin Cyr, 'Titanium in GGBS-like calcium-magnesium-aluminosilicate glasses: Its role in the glass network, dissolution at alkaline pH and surface layer formation', Journal of Non-Crystalline Solids 591 121708 (2022) doi:10.1016/j.jnoncrysol.2022.121708
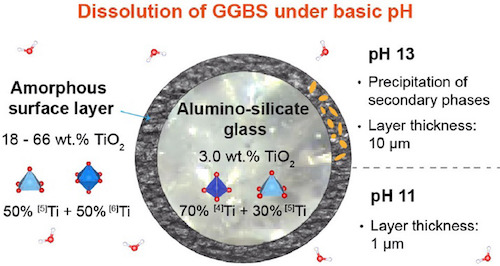
Ground-granulated blast-furnace slags (GGBS) are common replacements of Portland cement in low-carbon cements. However, small TiO2 contents in the slag-glass significantly reduce its cementitious reactivity. Here, we investigate the role of Ti in the glass network and its influence on slag-glass dissolution. XANES and EPR analysis showed that 67% of Ti was present as Ti(IV) with about 70 % in [5]Ti and 30 % in [4]Ti coordination and 33% as Ti(III). Initial dissolution rates at pH 11 were only slightly decreased (10-28%) by the presence of Ti in model glasses and modified slags, dissolved Si after 7d even less. During dissolution, Ti accumulated in an amorphous layer at the glass surface. At pH 11 the layer was mainly composed of TiO2 (66 wt.%), whereas it was intermixed with a hydrotalcite-like phase at pH 13. Ti K-edge XANES of the surface layer revealed a change of Ti coordination to 50% of [5]Ti and 50% of [6]Ti coordination at both pH. Our results suggest that the drastic loss of cementitious reactivity in the presence of Ti is not only due to stabilization of the glass structure by Ti, but also to the formation of a Ti-rich surface layer that may become passivating.Small TiO2 contents in the slag-glass significantly reduce their cementitious reactivity. We investigated the role of Ti in the glass network and its influence on slag-glass dissolution. XANES and EPR analysis showed that 67% of Ti was present as Ti(IV) with about 70% in [5]Ti and 30% in [4]Ti coordination, and 33% as Ti(III). The presence of Ti had only a minor impact on initial dissolution rates at pH 11 (R ≈ 10−6 molglass.s−1.m−2). During dissolution, Ti accumulated in an amorphous layer at the glass-surface. Ti-coordination in this layer was 50% [5]Ti and 50% [6]Ti. At pH 11, the layer was mainly composed of TiO2 (66 wt%), but intermixed with a hydrotalcite-like phase at pH 13. The loss of cementitious reactivity in the presence of Ti appears to be not only due to stabilization of the glass structure but also to the formation of a Ti-rich surface-layer, that may become passivating.
|
|