Ask for a reprint
email :
* Give your email
2021
ACL
|
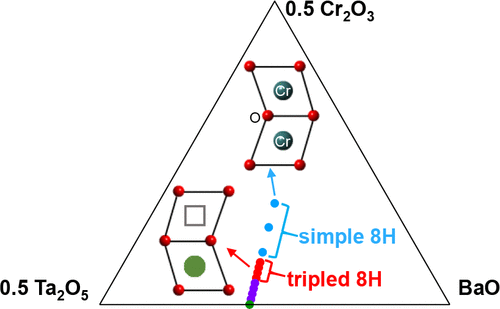
W.Cao, X.Yang, C.Genevois, M.Allix, X.Kuang, 'Extended B-Site Vacancy Content Range and Cation Ordering in Twinned Hexagonal Perovskites Ba8Cr4–xTa4+0.6xO24', Inorg. Chem. 60 3282-3290 (2021) doi:10.1021/acs.inorgchem.0c03707
Electrostatic repulsion between cations in face-sharing octahedral (FSO) sites is a key factor
controlling the hexagonal perovskite structure formation. Ba2CrTaO6 has been known for more
than 25 years as a line phase adopting an 8-layer twinned hexagonal perovskite structure with
chromium atoms in the FSO dimers forming semimetal Cr-Cr bonding, which decreases the FSO
B-B electrostatic repulsion. In this study, the aliovalent substitution of Ta5+ for Cr3+ in
Ba2CrTaO6 led to an 8-layer twinned hexagonal solid solution Ba8Cr4-xTa4+0.6xO24 (x =0.0-3.0)
containing FSO B-site vacancies. In this solid solution, the FSO B site occupancy varies from
full occupation to 15% vacancies, in great contrast with the analogue Ba8Ga4-xTa4+0.6xO24 (1.8 ≤ x
≤ 3.2), in which Ga-rich compositions close to the B-site fully-occupied end member Ba2GaTaO6
do not form 8-layer twinned hexagonal perovskite phases. Ba8Cr4-xTa4+0.6xO24 forms a simple 8-
layer hexagonal perovskite structure within 0.0 x < 2.4, and a tripled 8-layer hexagonal
perovskite superstructure within 2.4 x 3.0, with expanded a and b axes by compared to the
simple 8-layer hexagonal perovskite structure owing to the partial FSO B cation ordering along
the ab plane. The B-cation and vacancy distributions in the tripled superstructure were
characterized by neutron and X-ray powder diffraction and further confirmed by scanning
transmission electron microscopy-high angle annular dark field imaging and intensity profile
analysis. The formation of 8-layer twinned hexagonal perovskites Ba8Cr4-xTa4+0.6xO24 in an
extended solid solution range can be attributed to the presence of both FSO B-B and B-O-B
bonding and B-site vacancies. This work provides an effective way of combining B-B and B-OB
bonding and vacancy creation as well as the cationic ordering in FSO sites to reduce
electrostatic repulsion which could further enable the stabilization of new hexagonal perovskite
compounds.
|
|