Ask for a reprint
email :
* Give your email
2022
ACL
|
Ivette Aguilar, Pierre Lemaire, Nawfel Ayouni, Ezzoubair Bendadesse, Anatolii V.Morozov, Ozlem Sel, Véronique Balland, Benoît Limoges, Artem M.Abakumov, Encarnacion Raymundo-Piñero, Aneta Slodczyk, Aurélien Canizarès, Dominique Larcher, Jean-Marie Tarascon, 'Identifying interfacial mechanisms limitations within aqueous Zn-MnO2 batteries and means to cure them with additives', Energy Storage Mater. 53 238-253 (2022) doi:10.1016/j.ensm.2022.08.043
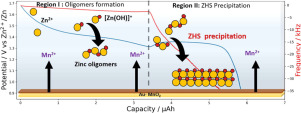
Li-ion batteries are playing a key role within the field of electrical mobility, grid applications and related objects owing to their high energy densities and long lifetime, but their sustainability remains to be improved. Pushing in this direction, there is a rising interest towards rechargeable aqueous batteries. Great efforts are devoted to turn the primary alkaline zinc-manganese dioxide batteries that have dominated the primary battery applications into rechargeable systems. This turns out to be a colossal task owing to the complexity of the Zn-MnO2 chemistry that is not yet fully rationalized, thus causing delay in practical deployment. In this work, we revisit this chemistry by combining fundamental solution chemistry considerations and complementary analytical techniques (TEM, Raman spectroscopy and EQCM) together with the assembly of cells using either MnO2 or MnO2-free initial positive electrodes. We confirm the key role of the electrolyte together with the inseparable link between its pH and the system's electrochemical response. Moreover, during discharge and charge, we provide experimental evidence for the occurrence of MnO2 electrodissolution and back electrodeposition conjointly with the formation of soluble zinc hydroxides up to chemical precipitation and back dissolution of a Zn4SO4(OH)6·xH2O phase. We show that this phase is essential in the buffering of the system's pH and demonstrate the beneficial role of its initial presence in the positive electrode composite. Further pushing the idea of buffering the pH of the electrolyte, we propose the use of additives such as ZnO, Mg(OH)2 or La(OH)3 that enhance the capacity retention upon cycling, while slightly penalizing the cell capacity. These insights provide missing links regarding the interplay between the conjoint electrochemical-chemical reactions ruling the functioning of rechargeable Zn-MnO2 batteries, hence providing a step forward towards their development.
|
|