Ask for a reprint
email :
* Give your email
2022
ACL
|
A.Zhadan, V.Sarou-kanian, L.Del Campo, L.Cosson, M.Malki, C.Bessada, 'Speciation and Transport Properties in Molten Alkali Carbonates at High Temperature', J. Phys. Chem. C 126 17234-17242 (2022) doi:10.1021/acs.jpcc.2c03048
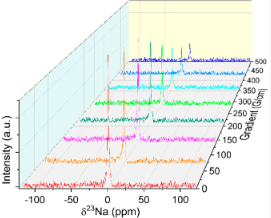
We study structural and transport properties of different eutectic mixtures of molten carbonate used for the capture and valorization of CO2. Different techniques, such as NMR, PFG NMR, and electrical impedancemetry, were used to study Li2CO3–Na2CO3 (52:48%mol), Li2CO3–K2CO3 (62:38%mol), Li2CO3–K2CO3 (40:60%mol), and Li2CO3–Na2CO3–K2CO3 (43.5:31.5:25%mol) compositions at high temperature. The NMR measurements confirmed only the presence of anionic species CO32–. No effect of composition or temperature on speciation was detected. From electrical conductivity and self-diffusion coefficients measurements, we have shown that the electrical conductivity of carbonates not only depends on the radius of the cations, but also depends on the electrostatic interaction between ions. With the addition of K2CO3 in the carbonate mixture, the interaction between the charge carriers present in the liquid increases and results in a decrease of electrical conductivity. We have shown that the high solubility of CO2 in compositions with high lithium carbonate content is associated with a high mobility of Li+ cations.
|
|