Ask for a reprint
email :
* Give your email
2024
ACL
|
M.Korenko, F.Šimko, M.Allix, A.Rakhmatullin, M.J.Pitcher, G.King, 'Determination of the Na3AlF6–Y2O3 Phase Diagram and Its Implications for Low-Temperature YAG/Nd:YAG Synthesis', Cryst. Growth Des. 24 7494 (2024) doi:10.1021/acs.cgd.4c00684
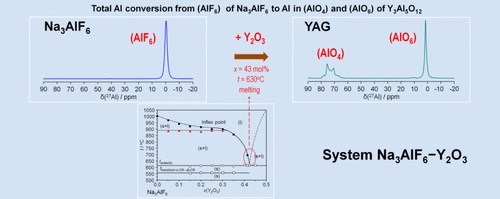
A new way of preparing YAG, Y3Al5O12, materials at low temperatures has been discovered using a molten Na3AlF6−
Y2O3 reaction mixture. For the successful synthesis of YAG, a precise examination of the cryolite part of the phase diagram of the
Na3AlF6−Y2O3 system was carried out up to 45 mol % of Y2O3 using thermal analysis with a larger amount of sample (12 g). The
phase diagram of the Na3AlF6−Y2O3 system was discovered to be likely a simple eutectic system with one inflection point on the
liquidus curve (coordinates: 22.0 mol % Y2O3, 920 °C) and one eutectic point (coordinates: 43.0 mol % Y2O3, 620 °C). The
spontaneously solidified samples of Na3AlF6−Y2O3 after thermal analysis have been investigated using solid-state NMR (19F, 23Na,
and 27Al) spectroscopy and X-ray powder diffraction over a broad range of compositions. The minimal synthesis temperature used in
this work for the preparation of YAG was 630 °C, and the Y2O3 concentration was 43 mol %. Besides the synthesis of YAG, the
molten Na3AlF6−Y2O3 system with the addition of Nd2O3 has been successfully used also for the preparation of the neodymiumdoped
YAG powders (Nd:YAG). Rietveld refinement has been used to quantitatively assess the incorporation of neodymium into
YAG and β-Na(Y1.5Na0.5)F6 materials.
|
|